Chimeric antigen receptor (CAR) T cell therapy is a type of immunotherapy that activates your immune cells (T cells) against sick or damaged cells.
In CAR T cell therapy, the patient’s own T cells are modified to add a special receptor (or hook), called a CAR, to their surface so they can find specific target cells like cancer.
Go to the home page to learn more.
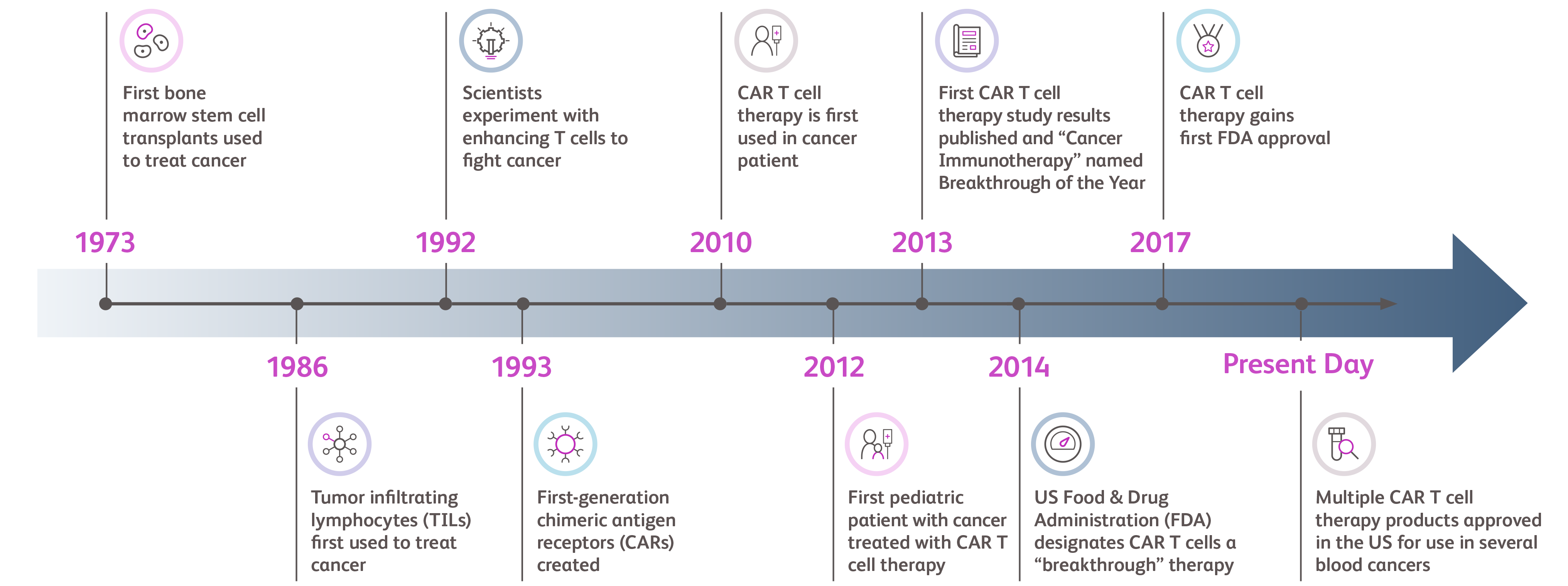
Researchers have been exploring ways to harness the immune system to combat cancer for decades. In 1993, the first CAR was created and since then, CAR T cell therapy has continued to be researched for over 30 years. Since its first FDA approval in 2017, CAR T cell therapies continue to demonstrate effectiveness and safety in clinical trials.
Beyond clinical trials, similar results have been seen in the "real world," meaning results have been collected from patients who received CAR T cell therapy after the treatment received FDA approval.
CAR T cell therapy may be used to treat specific types of leukemia, lymphoma, and myeloma.
For certain cancers, CAR T cell therapies may be used as early as after relapse or failure of initial treatment. Additionally, CAR T cell therapies are being investigated for the treatment of other disorders, such as solid tumors and autoimmune diseases.
CAR T cell therapy is an immune-based treatment that reprograms the patient’s own disease-fighting T cells to target cancer. These reprogrammed cells continue to multiply and attack cancer cells long after the infusion occurs.
Conversely, autologous stem cell transplant (ASCT) does not leverage the immune system to target cancer and instead may act as a “rescue” after a cancer treatment. ASCT uses your own stem cells (harvested from either your blood or bone marrow) to replace cells that have been destroyed by cancer therapy but does not target cancer directly.
Then, new healthy stem cells, from the bone marrow of the patient themselves, are given to the patient as a treatment.
Learn more about how CAR T cell therapy differs from ASCT and other cancer treatment options (PDF).
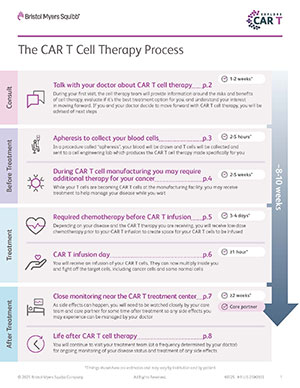
The typical length of time from consultation to treatment and recovery ranges from 8-10 weeks and may vary for each person.
The CAR T cell treatment itself is a one-time infusion.
Learn more about the steps involved in CAR T cell therapy and its timeframe (PDF).
During the CAR T cell therapy treatment journey, a patient may need additional support, such as transportation to appointments, household assistance, and emotional support. There is a wide network of people that may be part of a patient’s support team.
A care partner is critical to for personalized support through this process as needs may differ depending on the patient. Care partners can be a single person such as a spouse, family member, or paid care partner.
Additionally, patients may choose to have different care partners during different stages of the journey. They provide practical and emotional support throughout treatment, and they may help monitor for any symptoms or side effects.
CAR T cell therapy may cause side effects, which may mean that the CAR T cells are working to fight the disease. Care teams are trained to monitor for and manage side effects. Some early side effects may occur, which could feel like a bad case of the flu. Common symptoms to look out for include fever of 100.4°F or 38°C or higher, difficulty breathing, fast heartbeat, muscle or joint pain, headache, dizziness, and confusion.
Some patients may experience immune therapy-specific side effects. These include cytokine release syndrome (CRS), neurologic toxicity, and infections. The patient or their care partner should call for emergency help right away and call their doctor at the first sign of these side effects.
Read our Symptom Guide (PDF) for more information.
This does not encompass all the possible side effects of CAR T cell therapy, as they will vary from person to person based on a variety of factors, including what CAR T cell therapy product is prescribed.
Prior to treatment, patients should speak to their doctor about the possible side effects and when immediate medical care is necessary.
Patients who undergo CAR T cell therapy typically do not experience hair loss, which is a common side effect associated with chemotherapy.
If eligible to receive CAR T cell therapy, a doctor can refer patients to a qualified CAR T cell therapy center.
Staff at qualified treatment centers are trained on how to deliver treatment and support patients during every step of the process. Following CAR T cell treatment and side effect monitoring by the qualified CAR T cell therapy center, the patient can return to their referring physician for follow-up care.
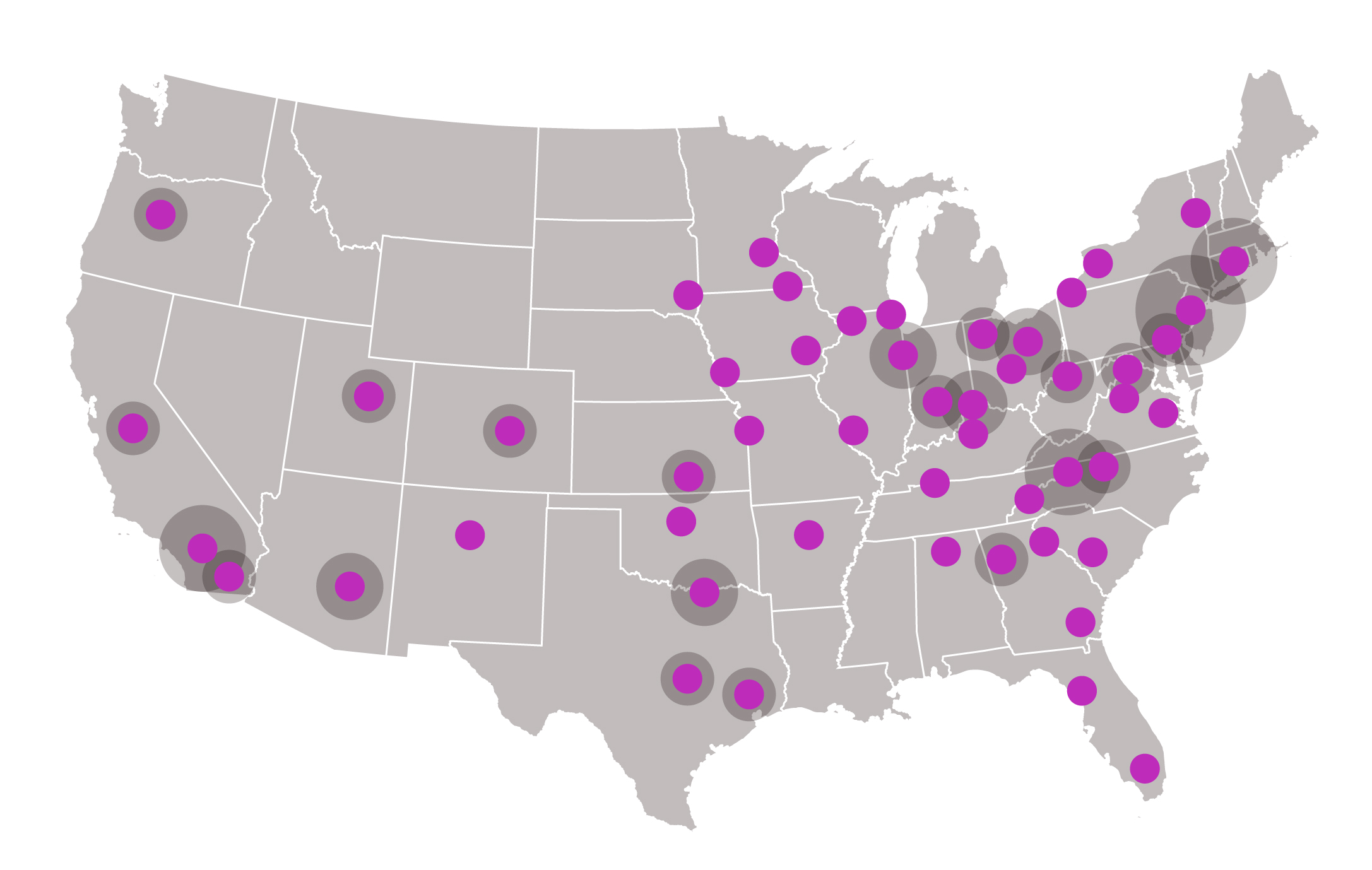
The number of qualified treatment centers continues to grow, with sites available around the U.S.
Check out this U.S. treatment center locator to find a center.
The majority of commercial insurance plans and most government payers cover CAR T cell therapies.
- For Medicare patients, CAR T cell therapies are covered for all FDA-approved indications under the National Coverage Determination
- The price most patients pay for CAR T cell therapies varies according to their insurance coverage and patient cost-sharing benefit design
- Additional assistance and support programs may be available from authorized treatment centers, product manufacturers, charitable foundations, patient financial support programs and other sources
Learn more about additional assistance and support programs.
